Borons forms the following types of organometallic compounds:
The alkyl or Aryl Borons, R3B.
Preparation: Trialkyl or triaryl boranes are prepared as follow:
1. BX3 + 3LiR → BR3 + 3LiX (R= alkyl or aryl group).
2. Actually, in this the reaction we get a mixture of BRX2, BR2X, and BR3.
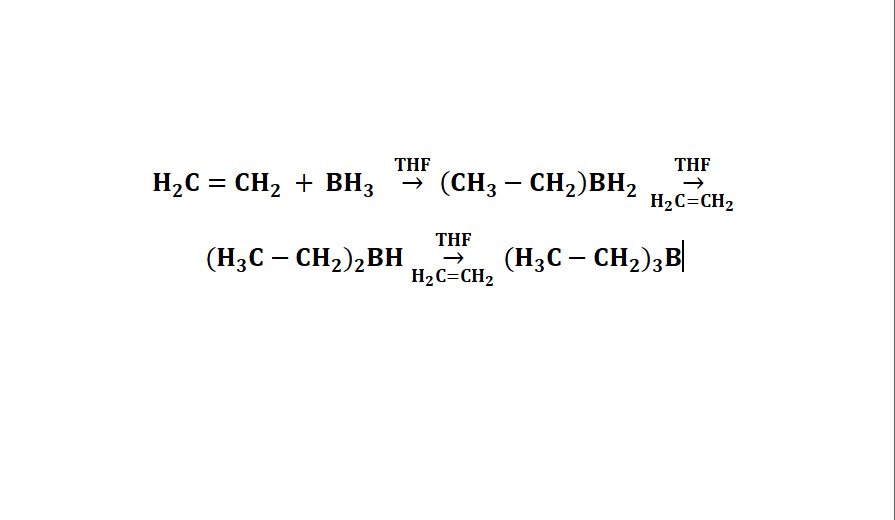
3. Trialkyl boranes can also be prepared, for example, from the reaction of 1-butene with diborane.
6CH3CH2CH=CH2 + CH2 + B2H6 → 2(CH3CH2 CH2CH)3B
Properties:
Trimethyl borane is a gas while triethyl and tri-n-propyl boranes are liquids and triphenyl borane is solid. The lower trialkyl boranes take fire spontaneously in air, the cautious oxidation of trialkyl boranes yield borate esters. Thus, oxidation with hydrogen peroxide yields a trialkyl borate.
R3B (Na+ C-H)B(OR)3
Triphenyl borane (C6H5)3B is not spontaneously inflammable, but is very easily oxidized and must be protected from air. Trialkyl and triaryl boranes are very reluctant to react with water and only at high temperatures are partially de-alkylated. At about 200°C, trimethyl borane is hydrolyzed to dimethyl borinic acid, (CH3)2B(OH)3:
(CH3)3B + 3H2O → (CH3)2B(OH)3 + CH4 + H2
Mixtures of alkyl boron halides arise when a trialkyl boron compounds is mixed with boron trihalides:
R3B + BX3 ⇌ R2BX + RBX2
Trialkyl boranes are monomeric. Since there are only three electrons in the valency shell of boron, trialkyl boranes are electron-deficient compounds. They form addition compounds with ammonia, amines and hydrazine.
Uses:
Alkyl and aryl borons are of great synthetic value.
Boronic and Boronous Acids
Boronic acid is B(OH)2R and boronous acid is B(OH)R. These are prepared as follows:
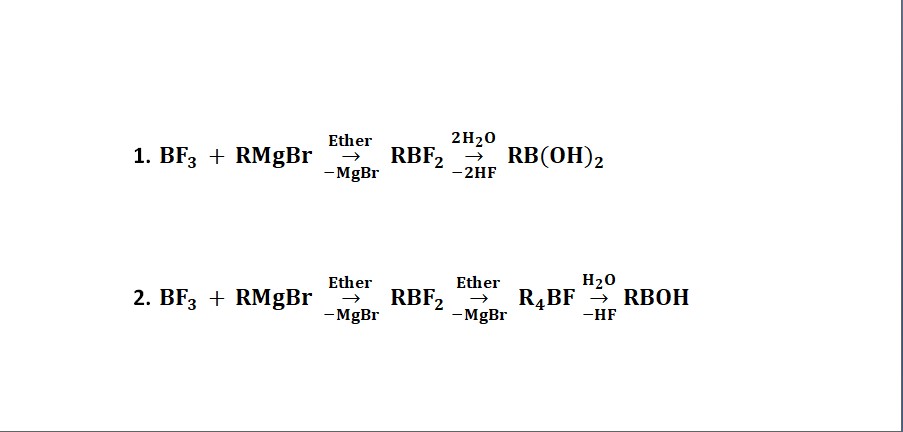
The strength of these acids depends upon the nature of R group.
Boronic acid is stable in water but on warming it polymerizes into a cyclic trimer.
Salts of M+ [BR4]– Type
Preparation:
1. Trialkyl borons (R3B) react with alkyl lithium (RLi) to form the salts of M+ [BR4]– type.
R3B + RLi → Li+[BR4]–
2. Li+ [B(C6H5)4]– is prepared as follows:
(C6H5)3B + Li C6H5 → Li+ [B(C6H5)4]–
Properties: Li+ [B(C6H5)4]– is insoluble in non-polar solvents but soluble in alcohol and water. The sodium salt, Na+[B(C6H5)4]– is used as an analytical reagent for the gravimetric estimation of heavy alkali metal ions (K+, Rb+, and Cs+) and a number of other cations.
Na+[B(C6H5)4]– can cause complete precipitation of K+, Rb+, and Cs+ from aqueous solutions of their salts
Structure:
The [R4B]– anion is tetrahedral in shape, with a complete octet around boron. It is isoelectronic with R4N+ and R4C.

