What is SN2 Mechanism?
SN2 stands for substitution nucleophilic bimolecular. This is a one-step process without forming any intermediate and hence it is a case of direct displacement reaction. In SN2 substitution the nucleophile attacks and forms a bond with it. This results in the formation of a transition state. Attack of nucleophile and departure of leaving group takes place simultaneously and the energy released by the formation of carbon nucleophile bond partly compensates the energy required for simultaneous breaking of carbon leaving group.
For this reaction, the reaction is also designated by ANDN where AN indicates the addition of nucleophiles and DN stands for departure or dissociation of nucleofuge (the species that leaves with electron ). Since the breaking of this original bond and formation of the new one are converted or simultaneous processes, this mechanism is also called a one-step mechanism and since the rate of reaction depends on the concentration of both the reactants, the reaction becomes bimolecular and corresponds to the second-order kinetics.
The entire picture is shown below.
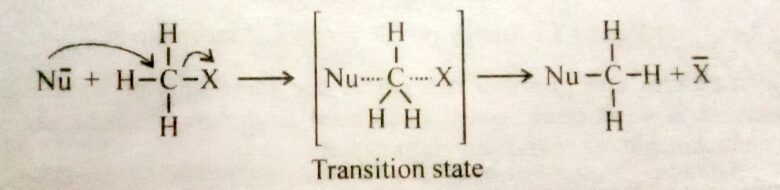
The reaction between methyl iodide and hydroxide ion is an example of an SN2 mechanism. The rate of such reaction is given by
Rate = k [CH3X] [Nu-]
Where k is a rare constant and quantity in square brackets represents concentration. The initially sp3 hybridized carbon atom becomes sp2 hybridized in the transition state, and the Nu- and X is associated with the two lobes o the unhybridized p orbital that is thereby made available. The approach of the nucleophile from the side of the molecule bearing the leaving group is unfavorable due to electrostatic repulsion and also due to steric factors. In the transition state for this reaction, the three non-reacting substitutes on the carbon lie in a plane with the carbon undergoing reaction. This plane is between the incoming and outing groups.
The Energy Diagram For SN2 Reaction
Shows the reactants being converted to products by way of one transition state, with no energy minimum representing a reactive intermediate. The reactant and products are separated by the high energy point of the reaction, the transition state. The energy difference between reactants and transition is called the activation energy and is related to the rate of the reaction. Higher the activation energy, the slower the reaction.
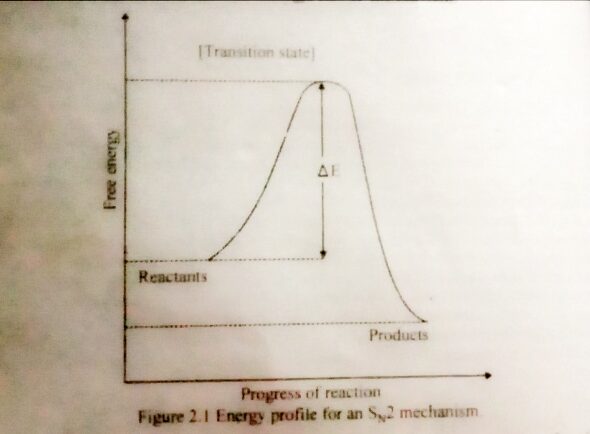
The reactants and products are not at the same energy level therefore, the forward reaction is exothermic as read from the left to right, and the backward reaction is endothermic, as read from right to left.